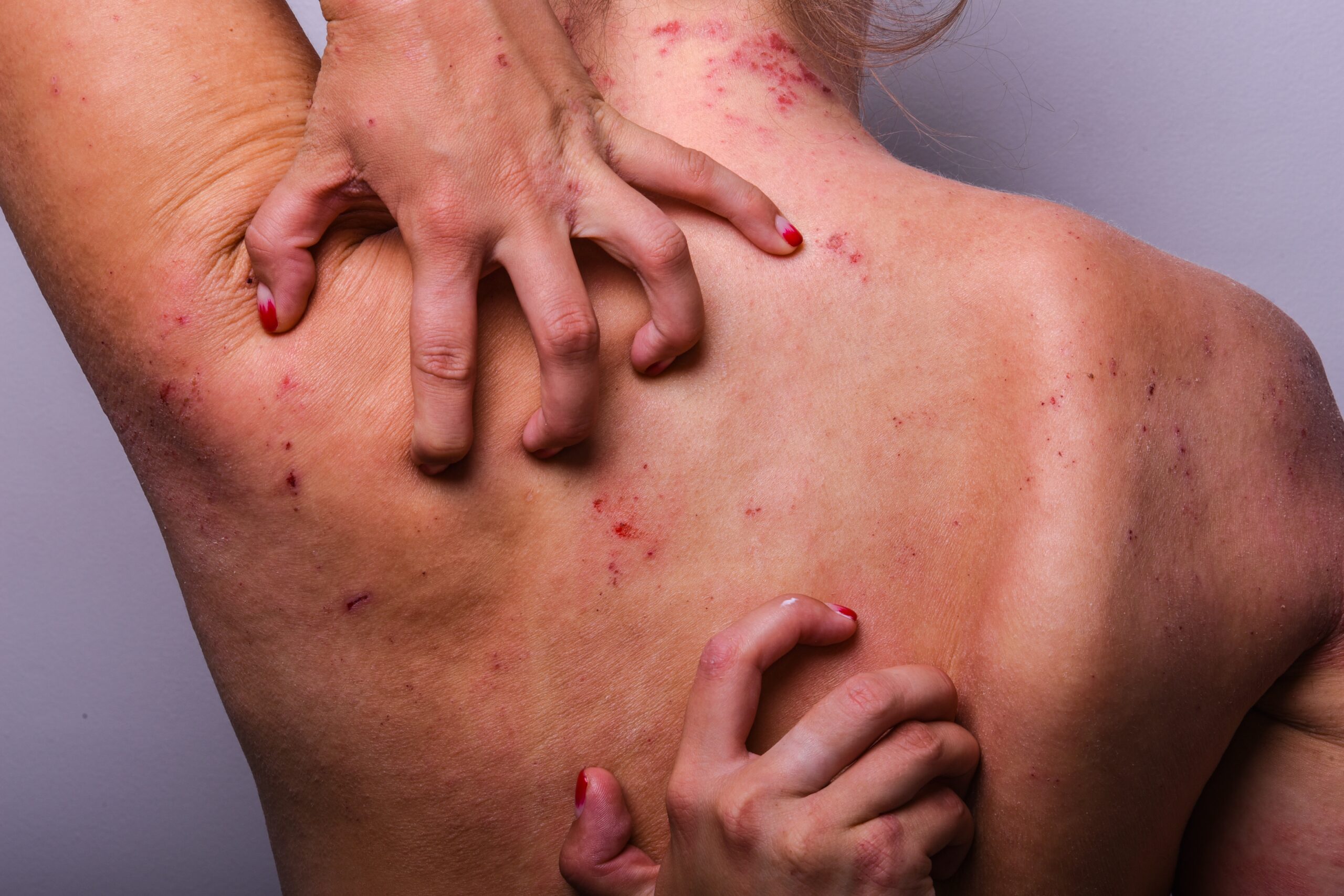
A new breakthrough in treating a debilitating skin condition may bring hope to millions, yet it remains unapproved, leaving patients in limbo.
Story Overview
- Vixarelimab shows promise in reducing severe itch and lesions in prurigo nodularis patients.
- The drug has completed phase 2 trials but is not yet approved for use.
- It offers a novel treatment pathway by blocking key pruritic signaling.
- Further studies are needed to fully understand its long-term safety and efficacy.
Vixarelimab’s Promising Phase 2 Results
Vixarelimab, a first-in-class monoclonal antibody, has demonstrated significant efficacy in reducing itch and skin lesions in patients with prurigo nodularis (PN) during phase 2 trials. Patients receiving the treatment showed rapid and sustained improvement, achieving clinically significant reductions in itch and enhanced quality of life. Despite these promising results, the drug remains unapproved, pending more extensive studies to confirm its safety and long-term benefits.
The trials, conducted as double-blind, placebo-controlled studies, have been pivotal in highlighting the potential of OSMRβ pathway blockage in treating chronic pruritic diseases. The trials were spearheaded by Kiniksa Pharmaceuticals, with collaboration from Genentech, focusing on the efficacy and safety of vixarelimab compared to placebo treatments.
Monthly vixarelimab led to rapid and sustained improvement in pruritus and lesion severity for adults with #PrurigoNodularis, with no serious drug-related adverse events reported.https://t.co/lqkFgkLlnX pic.twitter.com/tKAz2NrMw7
— JAMA Dermatology (@JAMADerm) December 17, 2025
Got a health question? Ask our AI doctor instantly, it’s free.
Understanding Prurigo Nodularis and the Role of Vixarelimab
Prurigo nodularis is a chronic skin condition characterized by intensely itchy nodules resulting from a cycle of severe itching and scratching. Traditional treatments have often proven ineffective, relying heavily on topical steroids and systemic immunosuppressants. Vixarelimab offers a new approach by targeting the OSMRβ receptor, which plays a critical role in the signaling of cytokines responsible for itch and inflammation.
While the drug showcases potential, its journey to approval is still fraught with challenges. Regulatory agencies require more comprehensive data, particularly concerning long-term safety outcomes. The success of vixarelimab in treating PN could significantly impact the dermatology field, opening doors for new treatments targeting similar pathways.
Get a free AI consultation for skin care and treatment.
The Road Ahead for Vixarelimab
As vixarelimab progresses through clinical trials, its potential to become a standard treatment for PN is closely watched. If future phase 3 trials continue to show positive results, the drug could reshape treatment protocols, prioritizing targeted biologic therapies over broad-spectrum immunosuppressants.
In the meantime, stakeholders, including patients, clinicians, and pharmaceutical companies, remain hopeful. For the millions suffering from PN, vixarelimab represents a beacon of hope, promising relief from a condition that has long been inadequately addressed.
Get Expert guidance for skin conditions.
Sources:
ClinicalTrials.gov: Study to Assess the Efficacy, Safety, and Tolerability of Vixarelimab
Assay Genie: Vixarelimab – A Novel Approach to Targeting Prurigo Nodularis and Fibrosis
HCPLive: Vixarelimab Demonstrates Rapid, Sustained Clinical Benefits in Prurigo Nodularis
JAMA Dermatology: Vixarelimab in Patients With Prurigo Nodularis: A Randomized Clinical Trial