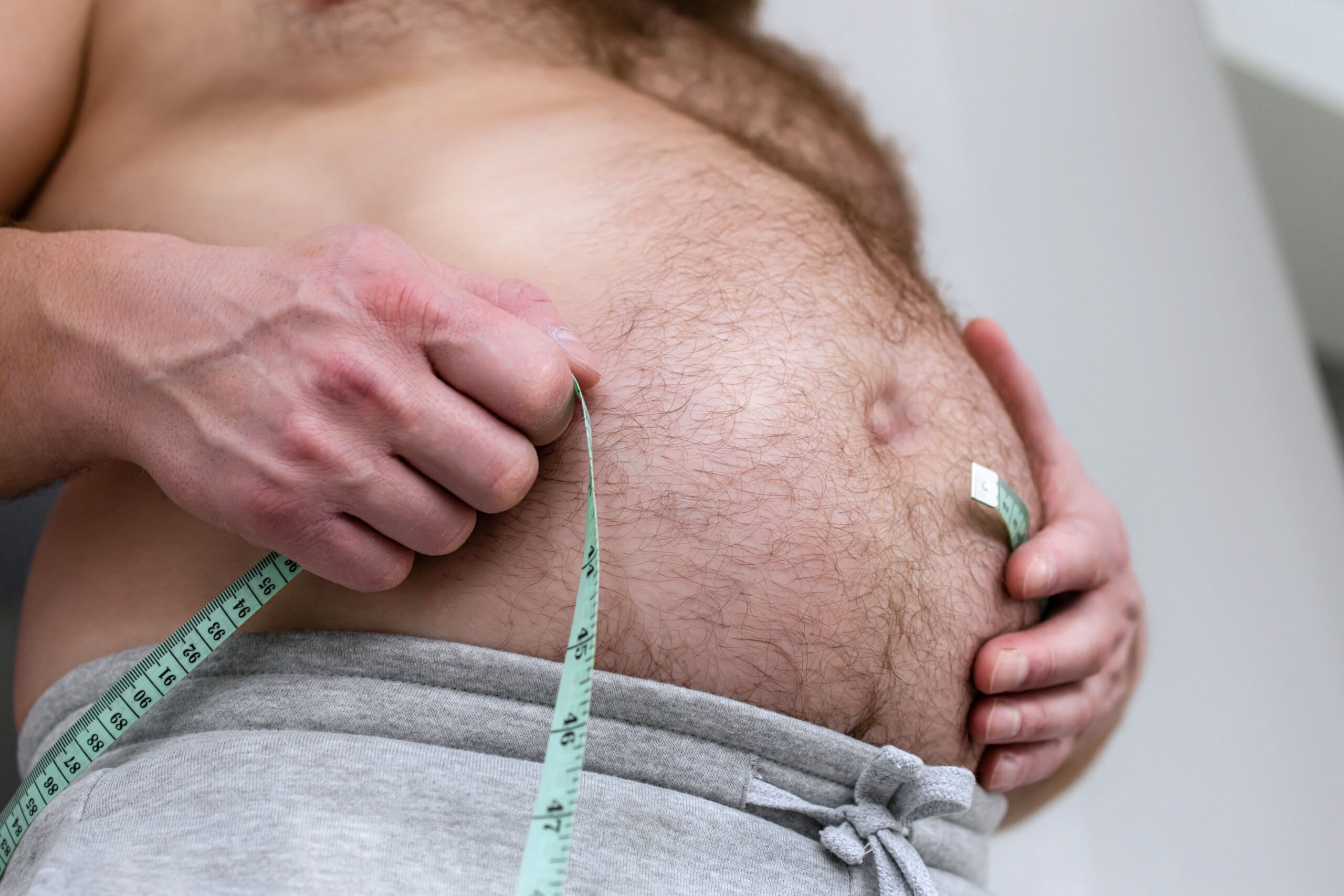
An innovative weight-loss pill is set to hit the market, but not everyone is thrilled about its implications.
Story Snapshot
- Novo Nordisk’s oral Wegovy pill approved by FDA for obesity treatment.
- First FDA-approved oral GLP-1 medication, transforming obesity care.
- Trump administration’s new RX platform to distribute the pill widely.
- Cost, prescription barriers, and competition remain challenges.
FDA Approval Sets New Precedent for Obesity Treatment
The FDA’s approval of Novo Nordisk’s oral Wegovy pill on December 22, 2025, marks a significant moment in obesity treatment. This oral GLP-1 medication, targeting adults with obesity or weight-related conditions, is the first of its kind and aims to provide a more accessible option for those averse to needles. The drug’s efficacy parallels its injectable counterpart, promising an average weight loss of 16.6% over 64 weeks. However, it is not without its challenges, including potential side effects and the necessity of a prescription.
While the pill offers an innovative approach, it arrives amidst a competitive landscape. Eli Lilly is advancing its own oral GLP-1 drug, orforglipron, which is expected to hit the market in 2026. The introduction of these oral options signifies a shift in how obesity is managed, potentially increasing adherence to treatment plans due to the convenience of a pill form. Despite the promise, questions around cost and insurance coverage remain, emphasizing the importance of monitoring economic impacts on patients and healthcare systems.
Watch:
Trump Administration’s Role in Distribution
The Trump administration has played a pivotal role in the distribution of the Wegovy pill through its newly launched RX platform, set to commence in early 2026. This collaboration aims to enhance access to the medication, promising to reach millions of Americans. The administration’s involvement highlights the political dimensions of healthcare accessibility, underscoring its commitment to expanding treatment options for obesity, a significant public health issue affecting millions nationwide.
The anticipated rollout aligns with Trump’s broader healthcare strategy, focusing on patient-centric solutions and reducing barriers to treatment. However, the initiative must navigate the complexities of healthcare costs and prescription requirements, which may limit the pill’s reach to those most in need.
Implications for the Healthcare Market and Society
The introduction of the Wegovy pill is anticipated to have substantial effects on the healthcare market and society at large. Short-term, the pill’s accessibility could significantly reduce needle-related barriers, providing a more appealing option for individuals seeking weight management solutions. Long-term, this development may standardize oral treatments for obesity, potentially transforming the industry with projected sales reaching billions.
While the promise of these drugs is undeniable, stakeholders must address ongoing challenges, including cost management and broader accessibility. The success of this initiative will depend heavily on how well these factors are managed in the coming years, ensuring that the transformative potential of obesity treatment is fully realized.
Your instant doctor companion – online 24 hours a day.
Sources:
FDA Approval of Novo Nordisk’s Oral GLP-1 Marks a New Phase in Obesity Treatment
FDA Approves Wegovy Pill for Weight Loss
Eli Lilly’s Orforglipron Phase III Obesity Weight Maintenance Trial
Weight Watchers Expands Its Integrated GLP-1 Platform with Access to Wegovy Pill