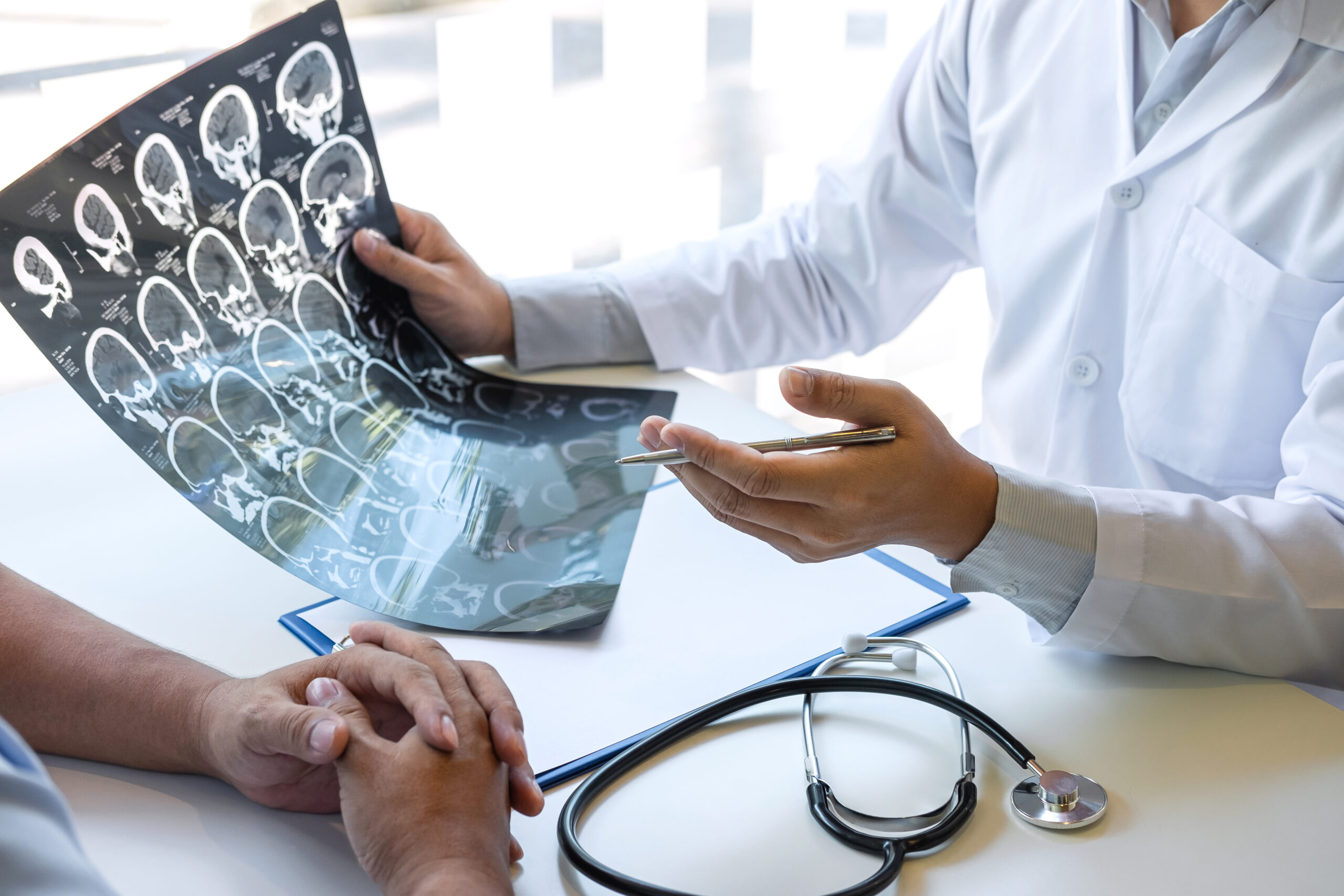
A dangerous brain tumor can spend years quietly laying its tracks through “normal” tissue before a scan ever shows a mass.
Story Snapshot
- South Korean scientists traced a common malignant brain tumor in younger adults to normal-looking glial progenitor cells in the cerebral cortex that pick up an IDH mutation.
- Those mutated cells can spread silently for years, creating a wide “pre-tumor” footprint that imaging can’t see.
- Spatial transcriptomics let researchers map where the mutant cells live and how they differ from healthy neighbors.
- The finding reframes recurrence: surgeons may remove the visible tumor while invisible mutant cells remain behind.
- Early-interception ideas follow fast, including detection tech and RNA-based drug concepts aimed at the earliest mutant cells.
The moment brain cancer stops looking “sudden”
IDH-mutant gliomas have always carried a cruel surprise: many patients look healthy until symptoms force an MRI, and by then a surgeon faces a visible mass surrounded by delicate brain real estate. Researchers in South Korea added a more unsettling layer. They found evidence that these tumors begin as normal-appearing glial progenitor cells in the cerebral cortex that acquire an IDH mutation and then spread—quietly—long before a radiologist can point to a tumor.
This matters because medicine has trained patients to trust the scan: no mass, no cancer. The new work challenges that comfort. It suggests a long preclinical phase where mutated cells look deceptively ordinary, seeded across tissue that still functions well enough to keep life moving.
What spatial transcriptomics revealed that imaging could not
The leap came from spatial transcriptomics, a method that reads gene activity while preserving a cell’s address in the tissue. That location detail is the game changer. Instead of treating the tumor as a single bad blob, scientists can map neighborhoods: which cells sit at the core, which linger at the edges, and which hide in tissue that looks normal under a microscope. In this case, the map pointed back to cortical glial progenitor cells carrying the defining IDH mutation.
That finding undercuts the simplistic idea that a glioma starts where the mass eventually appears. The research supports a “field” of mutated cells that can expand before forming a dense tumor. If that model holds across more patients, it helps explain why recurrence has been so stubborn. A surgeon can remove what is visible and still leave behind scattered mutant cells that never announced themselves on standard imaging.
Meet My Healthy Doc – instant answers, anytime, anywhere.
Brain cancer may begin years before doctors can see it – https://t.co/wjQQkDx1Wy
— Ken Gusler (@kgusler) January 29, 2026
Recurrence starts to look less like bad luck and more like biology
Patients and families often experience recurrence as betrayal: treatment works, the scan looks clear, then the cancer returns. The new origin story makes that cycle feel less mysterious. If mutant cells have already dispersed through the normal-looking cortex, a clean-looking post-surgical scan may reflect the limits of detection rather than the absence of disease. That reframing can be emotionally brutal, but it is also practical because it defines a target: the earliest mutant cells and the territory they occupy.
Early detection becomes the obvious next question, and it isn’t science fiction to ask it. Cancer research broadly has pushed “upstream,” chasing molecular signals before disease turns into a crisis.
The new arms race: detect earlier, treat smarter, monitor continuously
Several parallel efforts show where the field is headed. Teams are building detection strategies aimed at finding mutant signatures earlier, including approaches tied to hospital-based R&D and the broader multi-cancer early detection movement. Others are pushing treatment concepts that try to outmaneuver recurrence rather than chase it.
Examples include gene-therapy programs moving toward trials for glioblastoma, and virotherapy strategies designed to deliver tumor-killing genes and stimulate immunity—different tumor subtype, same obsession with durability. Monitoring is also getting a makeover. Bio-inspired implant concepts and advanced analytics aim to track tumors in real time, reducing the “wait and hope” gap between scans.
Got a health question? Ask our AI doctor instantly, it’s free.
The promise, the risk, and the sane way to think about it
Earlier detection sounds like automatic progress until you consider what a positive result means. Brain surgery and radiation are not casual responses. A screening tool that flags a risk years ahead of a visible tumor would need extraordinary accuracy, clear thresholds, and a real plan for what to do next.
Practical optimism fits best here. The South Korean findings sharpen the biological target: IDH-mutant cells in a defined cortical lineage, spreading before the mass forms. That clarity can guide better tests and smarter drugs, including RNA-based concepts aimed at halting progression early.
The haunting takeaway is also the hopeful one. If brain cancer can start years earlier than doctors can see, then the real battleground shifts to those unseen years—where the right detection and the right intervention could keep a “normal” brain normal.
Your instant doctor companion – online 24 hours a day.
Sources:
https://blog.dana-farber.org/insight/2026/01/ten-cancer-related-breakthroughs-giving-us-hope-in-2026/
https://www.sciencedaily.com/releases/2026/01/260128075350.htm
https://braintumourresearch.org/blogs/latest-news/blog-new-trial-for-brain-tumour-patients-to-start-in-early-2026-what-is-it-and-how-will-it-work
https://www.aacr.org/blog/2026/01/08/experts-forecast-cancer-research-and-treatment-advances-in-2026/
https://www.jefferson.edu/news/2026/01/jan-2026-roundup.html
https://www.masseycancercenter.org/news/were-aiming-for-a-cure-massey-and-vimm-researchers-achieve-potential-breakthrough-in-brain-cancer-treatment/
https://endbraincancer.org/2026/01/brain-cancer-clinical-trial/
https://braintumor.org/news/national-brain-tumor-society-announces-2026-cern-robert-connor-dawes-scientific-fellowship-awardee/
https://www.insideprecisionmedicine.com/topics/oncology/origins-of-brain-cancer-where-idh-mutant-gliomas-begin/